1. Firstly, Nitrogen and Hydrogen (the raw materials), are scrubbed, or cleaned,
to remove any impurities.
2. The mixture of Hydrogen and Nitrogen is compressed, until the pressure reaches
200 atmospheres.
3. The compressed gases now flow to the converter. In the converter, beds of iron
are kept at 450 degrees celcius. Iron is used as a catalyst, since this is a reversible
reaction. Iron forces the reaction to the right, creating ammonia. Only 15 % of the total mixture forms
ammonia.
4. The mixture is then forced into a cooler. Ammonia condenses to a liquid, forming
at the bottom of the cooler, but the rest of gases are recycled to attempt to form more ammonia.
5. The liquid ammonia is collected.
Here is the symbol equation for the reaction: N2 (g) + 3H2 (g) = 2NH3 (g)
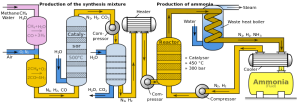
A very useful diagram, perhaps to understand the process a bit better!
The raw materials, Nitrogen and Hydrogen, are extracted from different sources. Nitrogen is
extracted from the air. Burning hydrogen in the air removes the oxygen leaving almost pure
Nitrogen. Hydrogen, however, is made by combining methane and steam, forming hydrogen.
As mentioned above, the reaction forming ammonia is a reversible reaction. This means: "Reactions
that do not go to completion and occur in both the forward and reverse direction". In other words,
if not mainted constantly, the reaction would keep reversing for ever. Therefore, the pressure,
temperature and catalyst play a key role in the reaction. Although this reversible reaction favours
lower temperatures, iron (the catalyst) needs at least 400 degrees celcius. The pressure levels are
then what force the equilibrium to produce more product. The iron lowers the activation energy of the
nitrogen and hydrogen, making the formation of ammonia easier. Without the iron, the equilibrium would
not be shifted. However, the reaction would go at such a slow rate, it would be silly to even attempt
the reaction.
